Osteoarthritis
- ACR Clinical classification criteria for Osteoarthritis of the hip
- ACR Clinical classification criteria for Osteoarthritis of the knee
- ACR Clinical classification criteria for Osteoarthritis of the hand
- ACR Guidelines for Medical Management of Osteoarthritis of the hip
- ACR Guidelines for Medical Management of Osteoarthritis of the knee
Rheumatoid Arthritis
- ACR Clinical Classification Criteria for Rheumatoid Arthritis
- ACR Clinical Classification Criteria for Juvenile Rheumatoid Arthritis
- ACR Classification Criteria for Determining Progression of Rheumatoid Arthritis
- ACR Classification Criteria for Determinining Clinical Remission in Rheumatoid Arthritis
- ACR Classification Criteria of Functional Status in Rheumatoid Arthritis
- ACR Guidelines for the Medical Management of Rheumatoid Arthritis Updated April, 2002
- ACR Definition of Improvement in RA trials
- ACR Recommendations for Monitoring Hepatic Safety in Rheumatoid Arthritis (RA) Patients Receiving Methotrexate (MTX)
Osteoporosis
- Osteoporosis Society of Canada Clinical Practice Guidelines for the Management of Osteoporosis
- ACR Guidelines for Prevention and Treatment of Glucocorticoid Induced Osteoporosis Updated July, 2001
Sjogren’s Syndrome
- EED Criteria for Diagnosis of Sjögren’s Syndrome
Pain Management
- American Geriatrics Society (AGS) Clinic Practice Guidelines forThe Management of Chronic Pain in Older Persons
ACR Clinical Classification Criteria for Osteoarthritis of the hip
Using history, physical examination and laboratory findings:
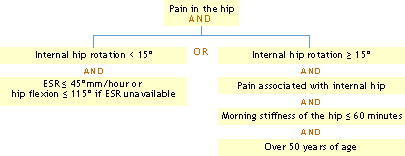
Using history, physical examination, laboratory and radiographic findings:
> Traditional format
> Classification tree
Reference: Altman, R, et al.: Arthritis Rheum 34:505, 1991
ACR Clinical Classification Criteria for Osteoarthritis of the knee:
Using history and physical examination
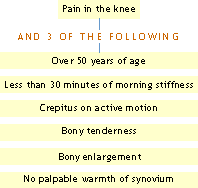
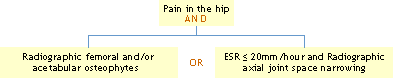
Using history, physical examination and radiographic findings:
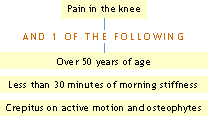
Using history, physical examination and laboratory findings:

Reference: Altman, R, et al.: Arthritis Rheum 29:1039, 1986.
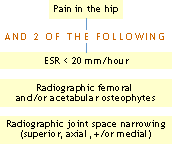
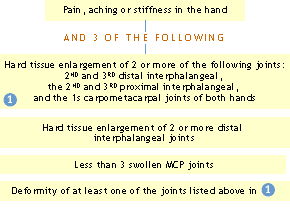
ACR Clinical Classification Criteria for Osteoarthritis of the hand
ACR Guidelines for Medical Management of Osteoarthritis of the hip
Updated 2000
Steps should be followed sequentially, moving to the next step if the patients’ response proves inadequate.

Reference: American College of Rheumatology Subcommittee on Osteoarthritis Guidelines: Arthritis Rheum 43(9):1905-15, 2000.
ACR Guidelines for Medical Management of Osteoarthritis of the knee
Updated 2000
Steps should be followed sequentially, moving to the next step if the patients’ response proves inadequate.

Reference: American College of Rheumatology Subcommittee on Osteoarthritis Guidelines: Arthritis Rheum 43(9):1905-15, 2000.
ACR Clinical Classification Criteria for Rheumatoid Arthritis
Using history, physical examination, laboratory and radiographic findings:
> Traditional format 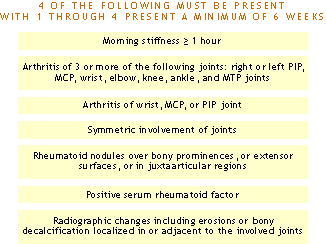
> Classification tree
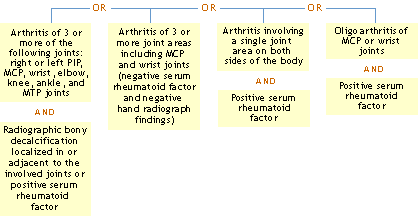
Reference: Arnett FC, et al.: Arthritis Rheum 31:315, 1988.
ACR Clinical Classification Criteria for Juvenile Rheumatoid Arthritis
GENERAL CLASS
a. Persistent arthritis of at least six weeks duration in one or more joints
b. Exclusion of other causes of arthritis (see list of exclusions+)
onset subtypes-determined by manifestations during the first six months of disease although manifestations more closely resembling another subtype may appear later
Systemic onset JRA*

subtypes:
Polyarthritis
Oligoarthritis
*Typical fever and rash will be considered probable systemic onset JRA if not associated with arthritis. Before a definite diagnosis can be made, arthritis, as defined must be present.
Pauciarticular*
![]()
subtypes:
Antinuclear antibody (ANA) positive-chronic uveitis
Rheumatoid factor (RF) positive
Seronegative, B27 positive
Not otherwise classified
*Patients with systemic onset JRA are excluded from this onset subtype.
Polyarticular
![]()
subtypes:
RF positivity
Not otherwise classified
*Patients with systemic JRA onset are excluded from this subtype.
+EXCLUSIONS
- Other rheumatic diseases
- Rheumatic fever
- Systemic lupus erythematosus
- Ankylosing spondylitis
- Polymyositis or dermatomyositis
- Vasculitic syndromes
- Scleroderma
- Psoriatic arthritis
- Reiter’s syndrome
- Sjogren’s syndrome
- Mixed connective tissue disease
- Behcet’s syndrome
- Infectious arthritis
- Inflammatory bowel disease
- Neoplastic diseases including leukemia
- Nonrheumatic conditions of bones and joints
- Hematologic diseases
- Psychogenic arthralgia
- Miscellaneous
- Sarcoidosis
- Hypertrophic osteoarthropathy
- Villonodular synovitis
- Chronic active hepatitis
- Familial Mediterranean fever
Reference: JRA Criteria Subcommittee of the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association Arthritis Rheum 20(Suppl)195, 1977
ACR Classification Criteria for Determining Progression of Rheumatoid Arthritis
*These criteria describe either spontaneous remission or a state of drug-induced disease suppression.

Reference: Steinbrocker O, et.al.: JAMA 140:659, 1949.
ACR Classification Criteria for Determinining Clinical Remission in Rheumatoid Arthritis
5 or more of the following present at least two consecutive months:
a. Morning stiffness < 15 minutes
b. No fatigue
c. No joint pain
d. No joint tenderness or pain on motion
e. No soft tissue swelling in joints or tendon sheaths
f. ESR (Westergren methold) < 30 mm/hour for a female or 20 mm/hour for a male
Exclusions: Clinical manifestations of active vasculitis, pericarditis, pleuritis or myositis, and unexplained recent weight loss or fever attributable to rheumatoid arthritis will prohibit a designation of complete clinical remission.
Reference: Pinals RS, et.al.: Arthritis Rheum 24:1308, 1981.
ACR Classification Criteria of Functional Status in Rheumatoid Arthritis
| Class I: | Completely able to perform usual activities of daily living (self-care, vocational, and avocational)* |
| Class II: | Able to perform usual self-care and vocational activities, but limited in avocational activities |
| Class III: | Able to perform usual self-care activities, but limited in vocational and avocational activities |
| Class IV: | Limited ability to perform usual self-care, vocational, and avocational activities |
*Self-care activities include dressing, feeding, bathing, grooming, and toileting. Avocational (recreational and/or leisure) and vocational (work, school, homemaking) activities are patient-desired and age- and sex-specific.
Reference: Hochberg MC, et.al.: Arthritis Rheum 35:498, 1992.
ACR Guidelines for Medical Management of Rheumatoid Arthritis (updated April, 2002)
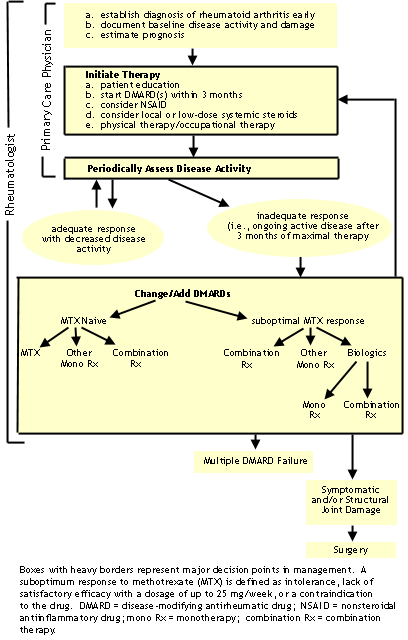
Reference: Arthritis & Rheum 46(2):328, 2002. Guidelines for the Management of Rheumatoid Arthritis. ACR Subcommittee.
©American College of Rheumatology. Reproduced with permission of Wiley Periodicals, Inc.
ACR Definition of Improvement in RA trials
In the past, the outcome criteria used in clinical trials to determine the effectiveness of new agents has varied making the comparison between agents and between clinical trials difficult. Two well accepted composite measures of improvement have emerged-the Paulus Criteria and The American College of Rheumatology Criteria (ACR). ACR criteria is rapidly becoming the current gold standard.
Paulus Criteria*

*The level of improvement is set as a percentage improvement of each of these variables i.e. a Paulus 20 classification indicates a responder who has shown 20% improvement in 4 of the 6 parameters.
The American College of Rheumatology Criteria (ACR) Criteria
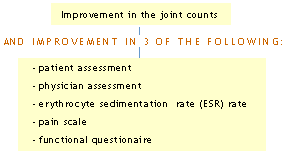
Improvement is denoted as either ACR 20, ACR 50 or ACR 70 reflecting either an improvement to the 20%, 50%, or 70% level in the parameters outlined. The ACR Success criteria (20, 50, 70) requires that the patient complete the trial and the patient meet ACR responder at the end of the trial.
ACR Recommendations for Monitoring Hepatic Safety in Rheumatoid Arthritis (RA) Patients Receiving Methotrexate (MTX)
1. Baseline
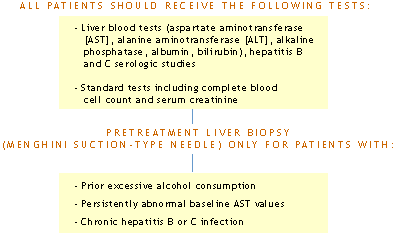
2. Monitor AST, ALT, albumin at every 4-8 weeks
3. Liver biopsy should be performed if:

4. If results of liver biopsy are:

5. Discontinue MTX in patient with persistent liver test abnormalities, as defined in 3, who refuses liver biopsy.
Osteoporosis Society of Canada Clinical Practice Guidelines for the Management of Osteoporosis
Comments by Dr. Michele Bellantoni
Diagnosis Recommendations
The Guidelines clearly recommend bone mineral density testing for individuals over age 50 years with at least one major risk factor or two or more minor risk factors. The Guidelines also clearly recommend that everyone over the age of 65 years should have a bone mineral density test. This statement is broader than the US Preventive Task Force Guidelines published in the Annals of Internal Medicine, Sept. 2003, that recommend universal testing only for women over age 65 years.
Risk Factors for Osteoporosis
| Major risk factors | Minor risk factors |
| Age >65 years | Rheumatoid Arthritis |
| Vertebral compression fracture | Past history of clinical hyperthyroidism |
| Fragility fracture after age 40 | Chronic anticonvulsant therapy |
| Family history of osteoporotic fracture (especially maternal hip fracture | Low dietary calcium intake (see nutritions section) |
| Systemic glucocorticoid therapy of >3 months duration | Smoker |
| Malabsorption syndrome | Excessive alcohol intake |
| Primary hyperparathyroidism | Excessive caffeine intake (see nutrition section) |
| Propensity to fall | Weight <57 kg |
| Osteopenia apparent on x-ray film | Weight loss >10% of weight at age 25 |
| Hypogonadism | Chronic heparin therapy |
| Early menopause (before age 45) |
These data in the table below provide physicians and patients with useful data on which to base clinical decisions for treatment.
Average 10-year Probability of an Osteoporotis Fracture*
| Age (years) | Overall average probability (%) | BMD expressed as T-score | ||||
| 1 | 0 | -1 | -2 | below -2.5 |
||
| Men | ||||||
| 50 | 3.3 | 1.8 | 2.7 | 4.2 | 6.3 | 9.2 |
| 55 | 3.9 | 1.9 | 3.0 | 4.6 | 7.0 | 10.4 |
| 60 | 4.9 | 2.5 | 3.6 | 5.4 | 7.9 | 11.6 |
| 65 | 5.9 | 3.0 | 4.3 | 6.2 | 8.8 | 13.0 |
| 70 | 7.6 | 3.4 | 5.1 | 7.4 | 10.9 | 16.2 |
| 75 | 10.4 | 4.1 | 6.3 | 9.6 | 14.4 | 21.5 |
| 80 | 13.1 | 5.3 | 7.7 | 11.1 | 15.8 | 23.2 |
| 85 | 13.1 | 5.3 | 7.5 | 10.4 | 14.3 | 21.4 |
| Women | ||||||
| 50 | 6.0 | 2.4 | 3.8 | 5.9 | 9.2 | 13.9 |
| 55 | 7.8 | 2.6 | 4.1 | 6.7 | 10.7 | 16.8 |
| 60 | 10.6 | 3.2 | 5.1 | 8.2 | 13.0 | 20.5 |
| 65 | 14.3 | 4.0 | 6.3 | 10.0 | 15.6 | 24.9 |
| 70 | 18.9 | 4.3 | 7.1 | 11.5 | 18.3 | 29.8 |
| 75 | 22.9 | 4.2 | 7.0 | 11.8 | 19.4 | 32.6 |
| 80 | 26.5 | 4.6 | 7.7 | 12.7 | 20.5 | 34.4 |
| 85 | 27.0 | 4.5 | 7.4 | 12.0 | 19.1 | 33.1 |
*wrist, hip, proximal humerus, vertebra.
Kanis et al. Osteoporosis Int 12:989, 2001.
Dietary Recommendations
It is encouraging to find that the Canadian guidelines recommend Vitamin D daily intake of 800 IU for men and women ages > 50 years. The US RDA continues to be 400 IU, though the literature supports 800 IU for the older adults, though the minimum age for the higher recommendation has not been well established.
The Canadian Guidelines explicitly do not recommend supplementation with the following nutrients: magnesium, copper, zinc, phosphorus, manganese, iron, and essential fatty acids. The guidelines correctly state that that no evidence exists to recommend supplementation with these nutrients. Many people spend excessive amounts of money on these types of nutritional supplements. The Canadian Guidelines differ in the recommendation of daily calcium intake for men ages 50-65 years from the current US RDA for adult men (1500 mg vs. 1000mg). I am not aware of evidence to support this recommendation.
Recommended Daily Intake
| CALCIUM | |
| Prepubertal children (ages 4-8) | 800 mg/day |
| Adolescents (ages 9-18) | 1300 mg/day |
| Women (ages 19-50) | 1000 mg/day |
| Women (ages over 50) | 1500 mg/day |
| Pregnant and lactating women (ages > 18) | 1000 mg/day |
| Men (ages 19-50) | 1000 mg/day |
| Men (ages over 50) | 1500 mg/day |
| VITAMIN D3 (Vitamin D2 shows less potency and more may be required to meet these recommendations) |
|
| Women (ages 19-50) | 400 IU (10ug/day |
| Women (ages over 50) | 800 IU (20ug/day |
| Men (ages 19-50) | 400 IU (10ug/day |
| Men (ages over 50) | 800 IU (20ug/day |
| ADDITIONAL NUTRIENTS | |
| Adequate protein intake is important | |
| Avoid excess caffeine (> 4 cups coffee/day) | |
| Avoid excess dietary sodium (> 2100 mg/day or > 90 mmol/day) as it reduces BMD in adult men and women. | |
| No evidence exists to recommend supplementation with the following nutrients for the prevention or treatment of osteoporosis: magnesium, copper, zinc, phosporus, manganese, iron, essential fatty acids | |
Treatment Recommendations
The Canadian Guidelines appropriately recommend oral bisphosphonates as first-line prevention and treatment of postmenopausal and glucocorticoid-induced osteoporosis, and in the treatment of men with osteoporosis. These Guidelines appropriately do not recommend oral bisphosphonate therapy for premenopausal women of child-bearing potential due to lack of evidence of safety. Additionally, the Guidelines appropriately recommend intranasal calcitonin as second-line treatment because of the lack of efficacy in the prevention of hip fractures in the large-scale clinical trials.
The Canadian Guidelines incorporate the results of the Womens Health Initiative Study in which no cardiovascular benefits of estrogen and progestin co-therapy were found to offset the risks of breast cancer and thromboembolic events of this therapy. The Guidelines also recommend estrogen as a second-line treatment, and caution about the risks of hormone replacement when used as a first-line preventive therapy. The Guidelines appropriately list the selective estrogen receptor modulator, raloxifene as first-line prevention and treatment of postmenopausal osteoporosis.
The Canadian Guidelines appropriately recommend against the use of Vitamin K and fluoride as treatments.
The Canadian Guidelines appropriately recommend parathyroid hormone therapy as first-line only for severe osteoporosis. The cost and potential side effects of the newly released preparation, hPTH (1-34), make this drug appropriate for use in extreme cases.
Optimal Treatment of Osteoporosis in Postmenopausal Women
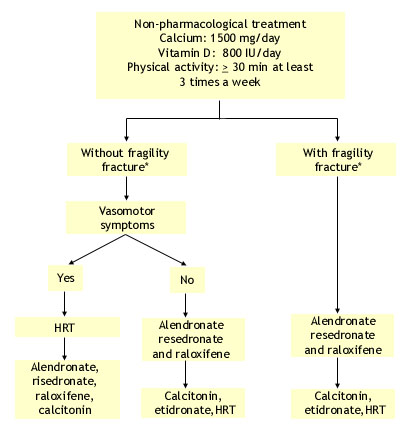
*mainly vertebral fracture. Only alendronate and risedronate and recently continuous estrogen-progesterone have been shown to decrease hip fracture risk.
Reference: JAMC 167(10 suppl), 2002
ACR Guidelines for the Prevention and Treatment of Glucocorticoid Induced Osteoporosis
Initial Evaluation

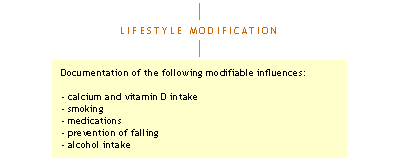

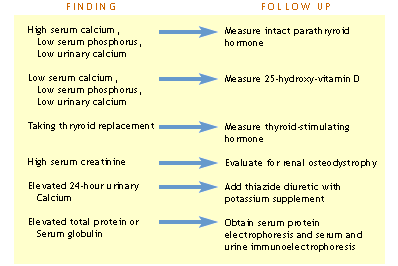
> Follow-up for abnormal laboratory findings
> Approach to the patient starting long-term glucocorticoid treatment

Recommendations for the Preventions and Treatment of Glucocorticoid-Induced Osteoporosis **Updated 2001
The following updated recommendations are the result of work by the ACR Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis published in Arthritis Rheum 44(7), 2001.
> Patient beginning therapy with glucocorticoid (prednisone equivalent of > 5 md/day) with plans for treatment duration of > 3 months.

> Patient receiving long-term glucocorticoid therapy(prednisone equivalent of > 5md/day)

Reference: Recommendations for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheum 44:1496-1503, 2001.
EEC Criteria for Diagnosis of Sjögren’s Syndrome
FOUR OR MORE OF THE FOLLOWING CRITERIA MUST BE PRESENT

Reference: Vitali C, Bomardieri S, Moutsopoulos HM. Preliminary criteria for the classification of Sjögren’s syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum 36, 1993.
AGS Clinical Practice Guidelines for The Management of Chronic Pain in Older Persons
Key Recommendations

Reference: American Geriatrics Society Clinical Practice Guidelines: The Management of Chronic Pain in Older Persons. Geriatrics 53(Suppl 3:S6-7), 1998.

